Get Patients with Peripheral Artery Disease Going
Medical Need
Current Treatment Options
- Exercise: no option in severe PAD
- Conservative Tx: risk factor reduction (e.g. BP reduction, glucose control, medication) – limited efficacy
- Intervention: Angioplasty or Vascular Surgery
- No-option patients: PAD stage IV – foot / leg amputation
Antepulsation
- Endogenous regeneration of biological bypasses (arteriogenesis)
- Non-invasive / non-surgical
- Personalized treatment PAD stage I – IV
- Bail out in PAD stage IV
Business Model
- Clear medical need
- Only enhanced external pro-pulsation on the market
- Experienced management
- Intellectual Property Protection
- Renowned clinical and financial advisors
- Low development costs through outsourcing
- Clear regulatory pathway for FDA / CE-Mark
- Existing reimbursement cases
- Dramatic growing market in the US and Europe
Get Patients Going
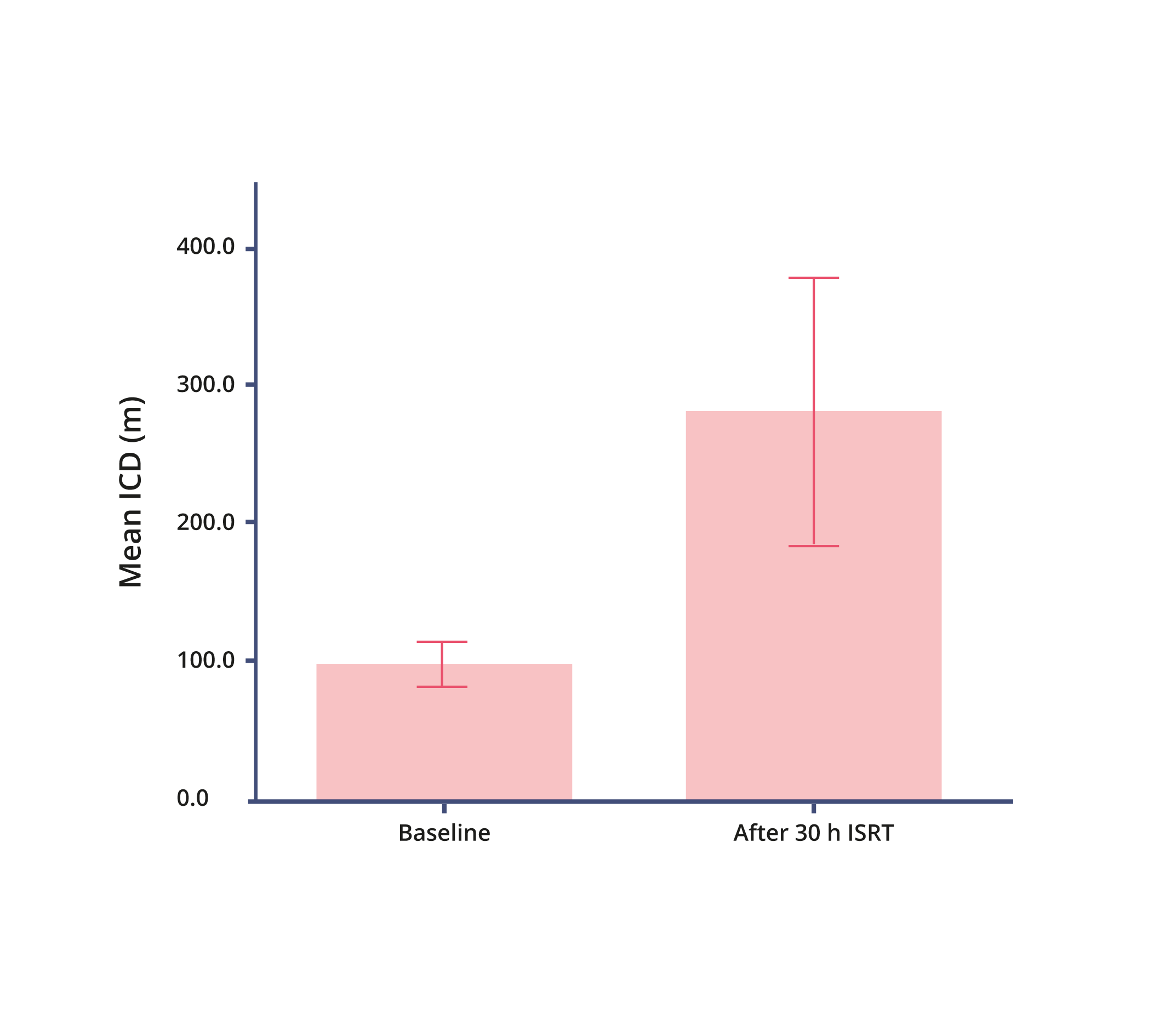
Initial Claudication Distance (ICD)
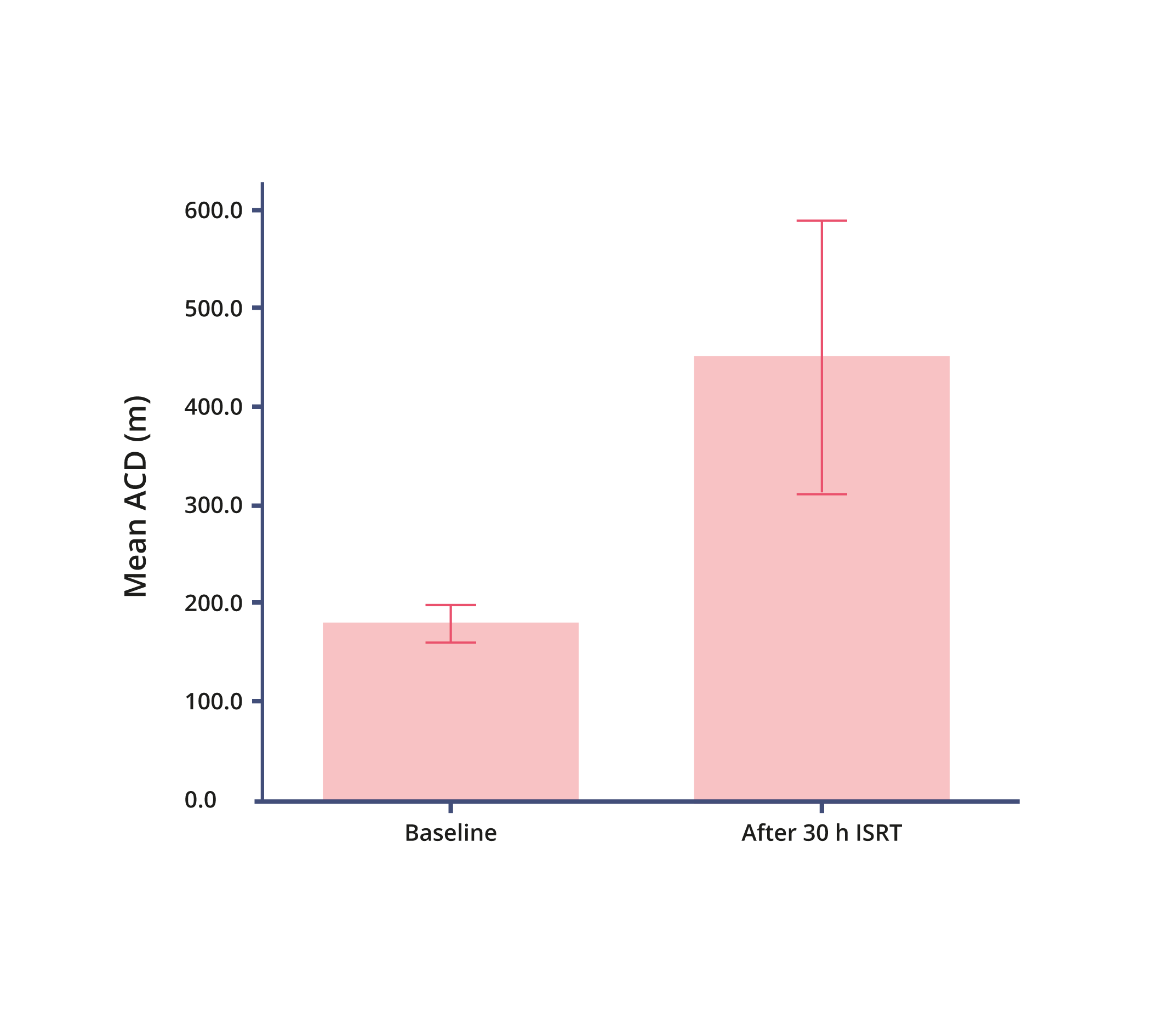
Absolute Claudication Distance (ACD)
Powered by People

Kai Ollrogge
CEO

Philipp Hillmeister, PhD
CSO
Our Achievements
- 2022-03-22: Antepuls GmbH receives the Validation Award 2022 from the Federal Ministry of Education & Research as part of its „AngioAccel“ research programme
- 2021-07-15: Antepuls GmbH receives a decision from the Federal Office of Economics and Export Control on the determination of eligibility for funding within the framework of Invest – Subsidy for Venture Capital
- 2021-06-21: Antepuls GmbH receives a grant of €50,000 from IBB Business Team GmbH as part of the „GründungsBONUS“ program.
- 2020-03-15: Antepuls GmbH officially founded to get patients with peripheral arterial disease going
- 2020-02-19: Clinical Study initiated
- 2018-06-19: First Closing of Seed Investment into improving the situation of 5 million people in the EU alone suffering from peripheral arterial disease (PAD)
Antepuls has been granted eligibility by the BAFA (Federal Office of Economics and Export Control) for INVEST funding. It is a program by the Federal Ministry for Economic Affairs and Energy to subsidize venture capital investments by private investors for young innovative companies. This government support follows the funding measure „Validation of the technological and societal innovation potential of scientific research – VIP+“ of the Federal Ministry of Education and Research (BMBF).
In Media
VIP + Validierungspreis 2022 – AngioAccel

7. April 2022, 14:43 – Copyright, Bildrechte, Quelle: ww.nachrichten.idw-online.de
Mit Druckluft gegen Durchblutungsstörungen. BMBF zeichnet MHB-Projekt AngioAccel aus
Das Therapiekonzept von AngioAccel erhöht mit Hilfe einer sogenannten ‚Herzhose‘ und EKG-gesteuerter Manschetten den Blutfluss von Patient*innen mit peripherer arterieller Verschlusskrankheit (pAVK) – einer Durchblutungsstörung in den Beinen, die vom Volksmund auch Schaufensterkrankheit genannt wird. Es konnte gezeigt werden, dass sich mit dem von Ärzten entwickelten Antepulsations-Verfahren die körpereigene Regeneration der Arterien und Durchblutungsstörungen in den Beinen auch nicht-invasiv sehr gut behandeln lassen. Das innovative Verfahren bietet insbesondere auch für bereits in ihrer Mobilität eingeschränkte Patient*innen einen deutlichen Vorteil gegenüber anderen Behandlungsmethoden.


